
A few days ago, the most recent research finding by Professor Geng Wu’s lab on protein complexes in the mTOR signaling pathway “Structural insight into the Ragulator complex which anchors mTORC1 to the lysosomal membrane” was published in Cell Discovery.
The mTOR signaling pathway us a very conserved signal transduction pathway present in unicellular organisms (such as yeasts), plants (such as Arabidopsis), invertebrates (such as fruit flies), and vertebrates (such as human). It regulates many important life processes such as cell growth and differentiation. Mutations of critical genes in the mTOR signaling pathway are closely associated with human diseases such as cancer and diabetes. The pentameric protein complex Ragulator is a key complex in the mTOR signaling pathway. It is located to the lysosomal surface through its Lamtor1 subunit. The Ragulator complex recruits the mTORC1 complex to the lysosomal surface, through the mediation of the RagA-RagC GTPases complex, to be activated by another small GTPase Rheb.
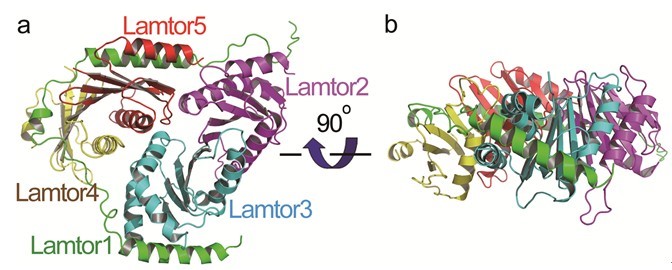
The researchers in Prof. Wu’s lab determined the crystal structure of human Ragulator. They found that one of its subunits, Lamtor1, wraps around the other four subunits Lamtor2, Lamtor3, Lamtor4, and Lamtor5 so as to assemble them together. The found that Lamtor4 and Lamtor5 lack the α3 helices present in Lamtor2 and Lamtor3. Strikingly, two helices from Lamtor1 fit into the grooves of Lamtor4 and Lamtor5 corresponding to the positions of the α3 helices of Lamtor2 and Lamtor3, so that the structures of Lamtor2 and Lamtor3 are stabilized. The have also found that the Ego complex in yeasts is only equivalent to half of the Ragulator complex: the C-terminal half of Lamtor1, Lamtor2, and Lamtor5, and they suggested that there might be additional undiscovered subunits of yeast Ego and more vigorous search was warranted.
This work is the third paper published by Prof. Wu’s lab on the structure and function of protein complexes in mTOR signaling pathway. In one of the previous two papers, they determined the crystal structure of the C terminal coiled coil of the tumor suppressor TSC1 in complex with TBC1D7, and revealed that TBC1D7 functions to stabilize the dimerization of TSC1’s C-terminal coiled coil (Gai et al., Journal of Molecular Cell Biology, 8:411-425, 2016). In the other work, they determined the crystal structure of CASTOR1, the arginine sensor of the mTOR signaling pathway, in complex with its ligand arginine. They elucidated the molecular mechanism of how CASTOR1 specifically recognizes arginine, and how CASTOR1’s conformational change induced by arginine binding relieves CASTOR1’s inhibition of its downstream GATOR2 complex (Gai et al., Cell Discovery, 2:16051, 2016).

Address: 800 Dongchuan RD. Minhang District, Shanghai, China
Phone: +86-21-34205709
School of Life Sciences and Biotechnology, SJTU Copyright © 2017 沪交ICP备05029. All Rights Reserved.


