
Recently, the team of Prof. Xinqing ZHAO from the School of Life Sciences and Biotechnology of Shanghai Jiao Tong University (SLSB, SJTU) published the latest results of the team’s research on xylose biosensors in ACS Synthetic Biology. The publication is titled Design, evolution, and characterization of a xylose biosensor in Escherichia coli using the XylR/xylO system with an expanded operating range. The first author of this article is Ruiqi TANG, a PhD candidate from Zhao Group, while Prof. ZHAO and Prof. Hal Alper from The University of Texas at Austin school are both the corresponding authors.
Biorefinery of lignocellulosic biomass is receiving increasing attention, which contributes to sustainable development of economy. Xylose can be released by hydrolysis of lig in nature. Using fermentable microorganisms, xylose can be converted to biofuels and various value-added chemicals. Due to the importance of xylose in lignocellulosic biorefinery, it is of great interest to develop efficient xylose-utilizing microbial cell factories.
Biosensors are useful tools that are widely used in synthetic biology, which commonly consist of three parts: 1) the sensing part; 2) the transduction part, and 3) the output part. Xylose biosensors reported in the previous studies exhibited limited operating range of xylose concentration compared to the xylose concentration in lignocellulosic hydrolysates, which limited their application in screening of recombinant microorganisms. Thus, it is of great importance to develop a xylose biosensor with an expanded operating range.
In the recent work reported by Tang et al., directed evolution was performed on a xylose regulator XylR, and xylose biosensors were developed based on the resultant XylR mutants and its operator xylO. After error-prone PCR and three-step FACS (fluorescence-activated cell sorting) of XylR mutants, the operating range of biosensor containing the mutant XylR was increased by nearly 10-fold comparing with the control. Specifically, two individual amino acid mutations (either L73P or N220T) in XylR were sufficient to extend the linear response range to upwards of 10 g/L xylose. The evolved biosensors described here are well suited for developing whole-cell biosensors for detecting varying xylose concentrations across an expanded range. This evolution strategy identified a less-sensitive biosensor, namely, a “bad” biosensor, for real applications, thus providing new insights into strategies for expanding operating ranges of other biosensors for synthetic biology applications.
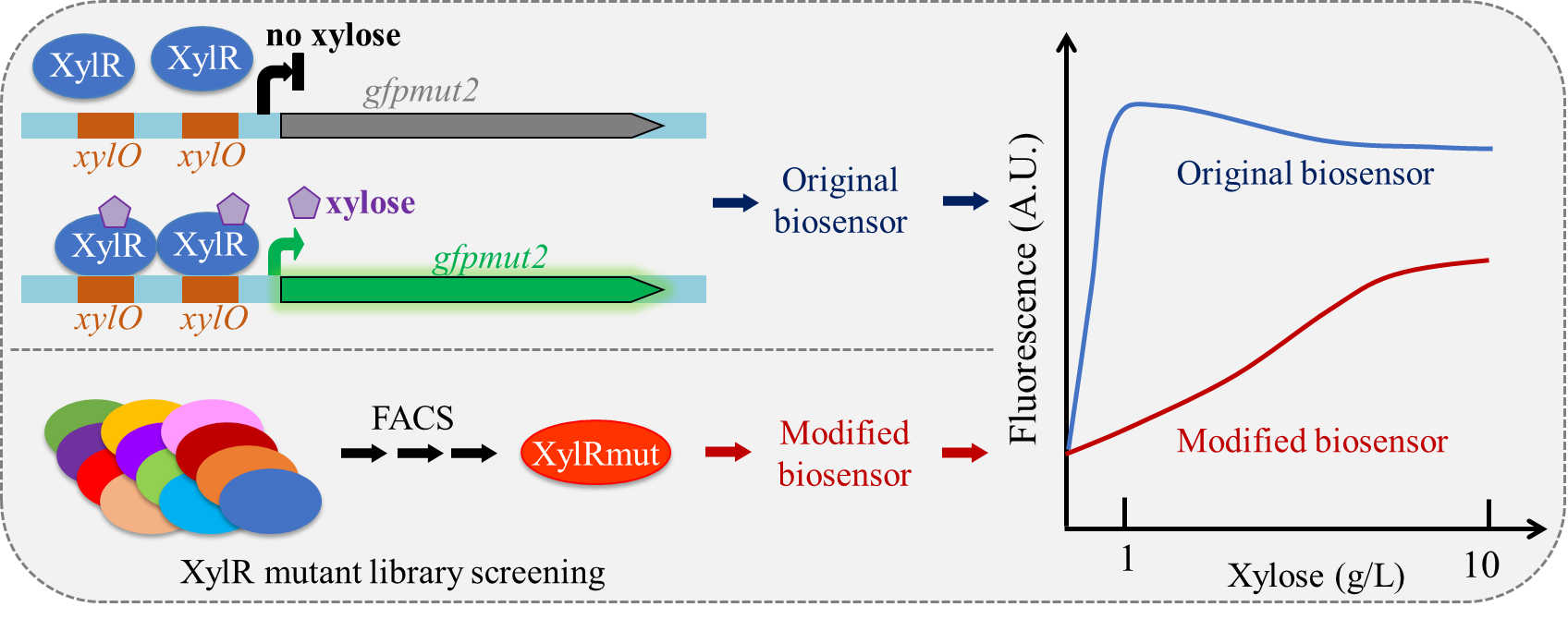
Figure: Novel xylose biosensor for strain screeing
This work was supported by National Science Foundation of China (No. 21536006), and the Open Project of the State Key Laboratory of Microbial Metabolism in China (MMLKF19-01). The full text link is https://doi.org/10.1021/acssynbio.0c00225

Address: 800 Dongchuan RD. Minhang District, Shanghai, China
Phone: +86-21-34205709
School of Life Sciences and Biotechnology, SJTU Copyright © 2017 沪交ICP备05029. All Rights Reserved.


