Recently, the latest research result of the team led by Associate Professor Xuanming YANG from School of Life Sciences and Biotechnology, titled "A chimeric antigen receptor with antigen-independent OX40 signaling mediates potent antitumor activity", was published on the website of Science Translation Medicine, an internationally renowned journal, and was labeled as recommended paper. The research found the antigen-independent OX40 signaling for the first time, which provides an applicable strategy for CAR-T optimization and overcoming the challenges in solid tumors treatment. Prof. Xuanming YANG is the corresponding author; doctoral students Huihui ZHANG and Fanlin LI from SJTU and Chief Physician Jiang CAO from Xuzhou Medical University are the co-first authors. Shanghai Longyao Biotechnology Co., Ltd. is responsible for the preparation of CAR-T for clinical treatment.
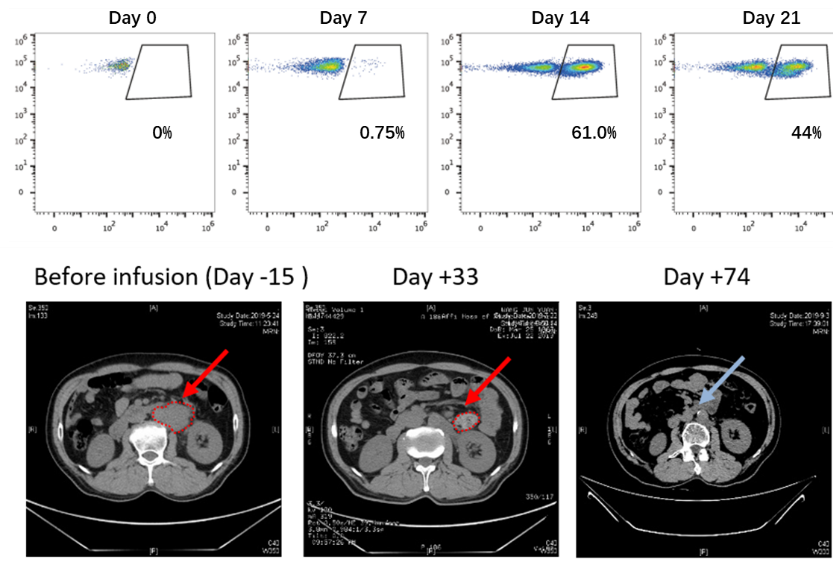
The researchers engineered a new type of CAR-T cell with costimulatory signal that was activated independently from the tumor antigen to recapitulate physiological stimulation of second-generation CAR-T cells. By comparing 12 kinds of costimulatory receptors, the researchers found that 20BBZ-OX40 CAR-T cells containing OX40 costimulatory receptors show superior proliferation ability, anti-apoptotic ability, and antitumor ability compared to BBZ CAR-T cells that are widely used in clinical treatment. Both anti-depletion ability and tumor killing ability have been improved. These characteristics have led to enhanced therapeutic efficacy in preclinical mouse tumor models and in patients with metastatic lymphoma. Their findings provide an alternative option for CAR-T optimization with the potential to overcome the challenge of treating solid tumors.
This work was funded by the National Key Research and Development Program (2016YFC1303400), the National Natural Science Foundation of China (81971467 and 81671643), and SJTU Science and Technology Innovation Special Fund.
ABSTRACT:
Although chimeric antigen receptor (CAR)-modified T cells have shown great success in the treatment of B cell malignancies, this approach has limited efficacy in patients with solid tumors. Various modifications in CAR structure have been explored to improve this efficacy, including the incorporation of two costimulatory domains. Because costimulatory signals are transduced together with T cell receptor signals during T cell activation, we engineered a type of CAR-T cells with a costimulatory signal that was activated independently from the tumor antigen to recapitulate physiological stimulation. We screened 12 costimulatory receptors to identify OX40 as the most effective CAR-T function enhancer. Our data indicated that these new CAR-T cells showed superior proliferation capability compared to current second-generation CAR-T cells. OX40 signaling reduced CAR-T cell apoptosis through up-regulation of genes encoding Bcl-2 family members and enhanced proliferation through increased activation of the NF-κB (nuclear factor κB), MAPK (mitogen-activated protein kinase), and PI3K-AKT (phosphoinositide 3-kinase to the kinase AKT) pathways. OX40 signaling not only enhanced the cytotoxicity of CAR-T cells but also reduced exhaustion markers, thereby maintaining their function in immunosuppressive tumor microenvironments. In mouse tumor models and in patients with metastatic lymphoma, these CAR-T cells exhibited robust amplification and antitumor activity. Our findings provide an alternative option for CAR-T optimization with the potential to overcome the challenge of treating solid tumors.



